Welcome to the RBDCOV Community Engagement Toolbox: a digital hub of resources developed by EATG and the partners of the RBDCOV Project. This toolbox brings together engaging, practical, and inclusive materials for activists, community members, researchers, and health professionals.
PENDING EUROPEAN COMMISSION APPROVAL
Welcome to the RBDCOV Community Engagement Toolbox: a digital hub of resources co-developed by EATG and members of the Community Advisory Panel within the EU-funded RBDCOV Project. This toolbox brings together engaging, practical, and inclusive materials for activists, community members, researchers, and health professionals interested in strengthening community participation and capacity in vaccine and broader clinical research.
The tools gathered here reflect EATG’s mission values: ensuring that the voices of people most affected by HIV and health inequalities are heard, respected, and central in the design of clinical research and health communication.
Every resource is rooted in equity, community engagement, and accessibility, helping you connect, learn, and participate within and beyond the scope of vaccine research.
This is a living toolbox: it is updated over time with new relevant materials as they are further developed, approved, or identified as useful.
For the creation of this toolbox, all third-parties online materials were accessed and retrieved in June and July 2025. EATG is not responsible for the accessibility of materials that are hosted outside of its servers. If a link is not working please contact us.
About the RBDCOV project
The RBDCOV project, which aimed to test a vaccine against COVID-19 in the paediatric population, including adolescents, and in immunocompromised individuals, plays a crucial role in vaccine development.
RBDCOV is one of the projects committed to testing and advancing a new vaccine against COVID-19. For this purpose, companies and institutions from five European countries have joined forces. The project is led by the biotechnological pharmaceutical company HIPRA and involves the participation of centres in Spain (Vall d’Hebron Institut de Recerca, Irsicaixa, Fundación Lucha contra el SIDA (FLS), IDIBAPS, IDIBGI, ASPHALION, Vinces Consulting, Zabala Innovation), the United Kingdom (Veristat International), Italy (Fondazione Penta), Germany/Belgum (European AIDS Treatment Group), and Turkey (Metpharm Arastirma Gelistirme Saglik Danismanlik).
To learn more about the RBDCOV project, start by watching this introductory video.
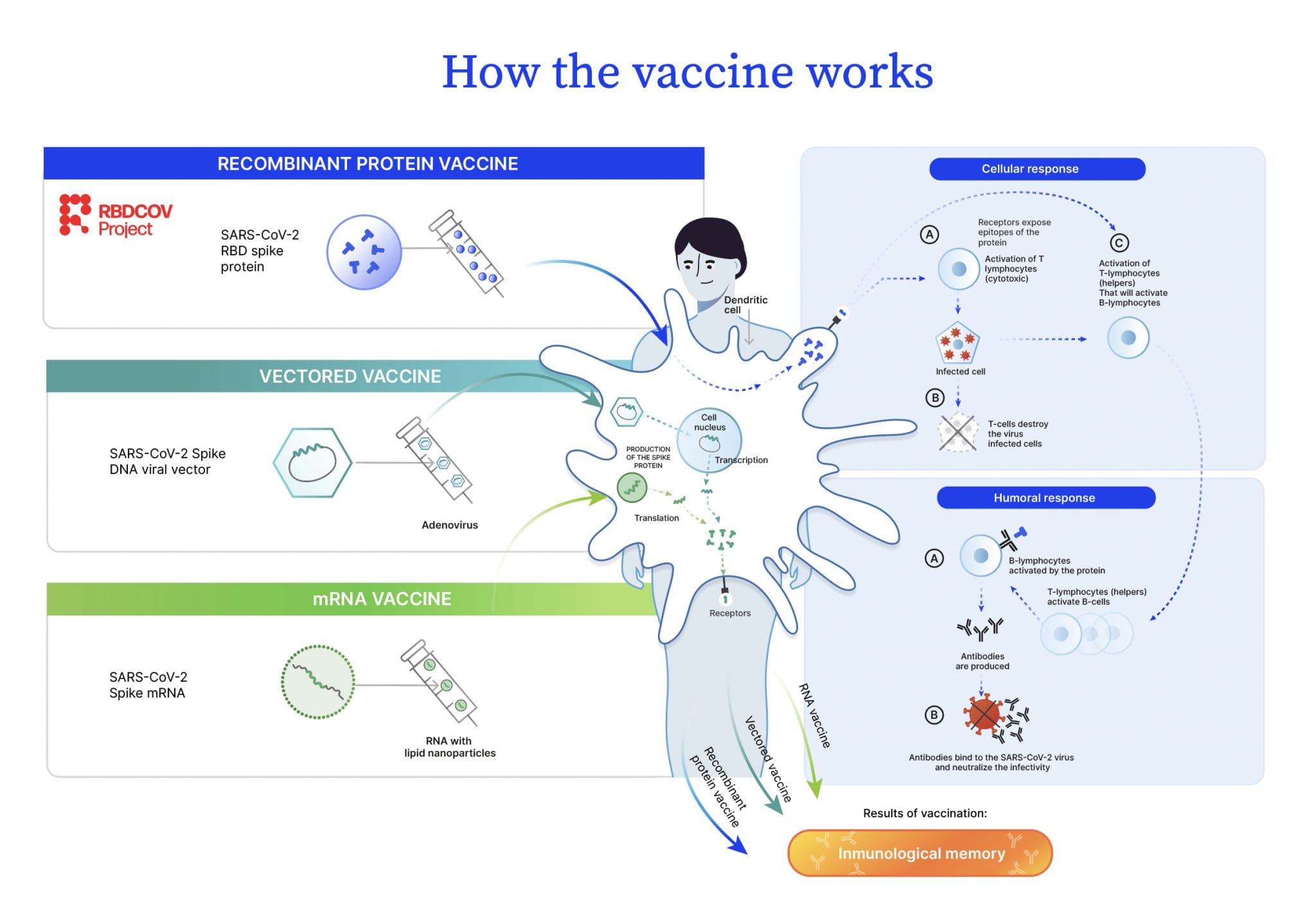
Curious about how the BIMERVAX® COVID-19 vaccine, a recombinant protein-based vaccine, works? Explore RBDCOV’s infographic for a clear and concise overview.


Informed Consent Forms and Participant Information Sheets for the RBDCOV Clinical Trials and Psychosocial Study

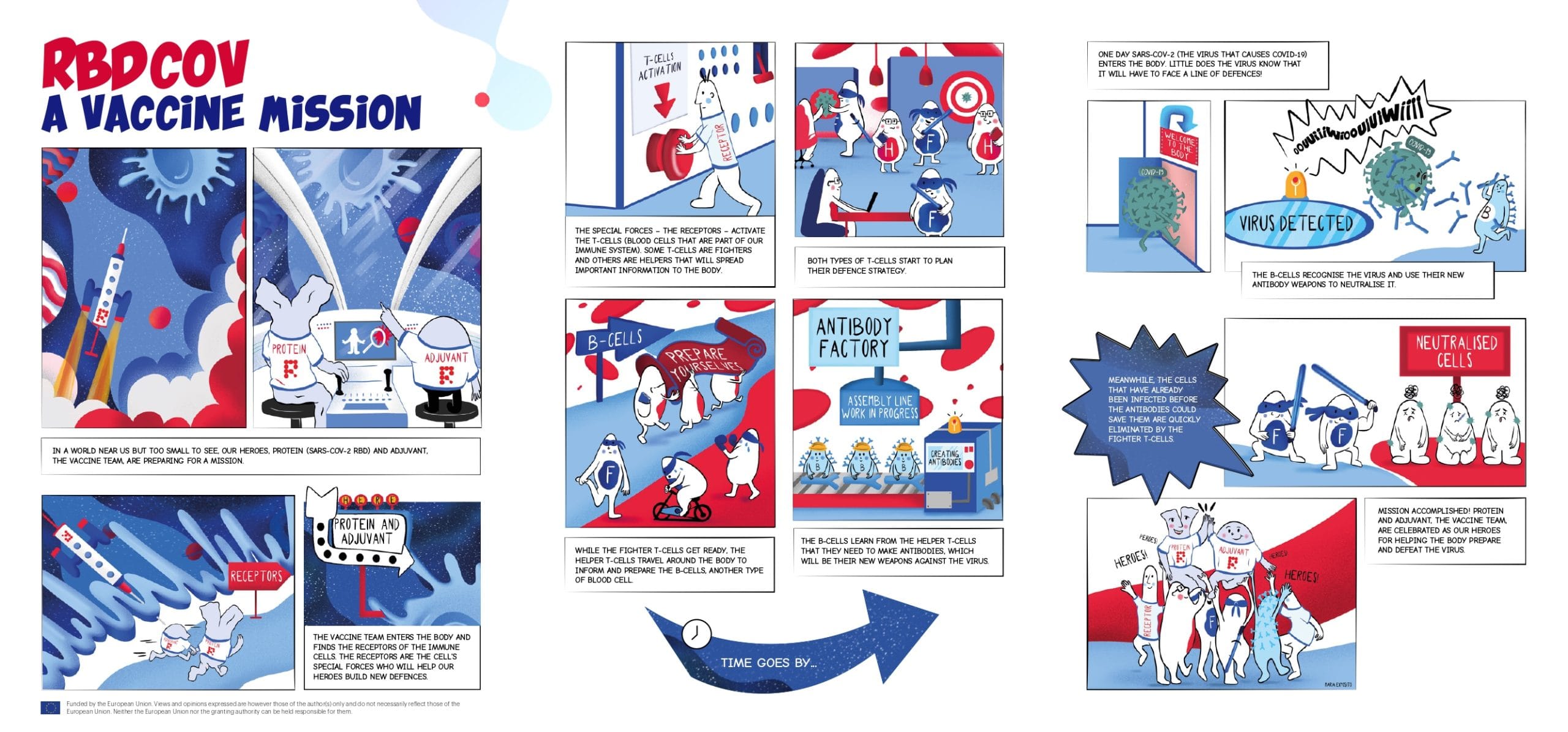
Comic Strip Infographic: RBDCOV: A Vaccine Mission

Inclusive HIV Terminology Guidelines: Considerations for internal and external communications

ReCAP Articles: Key Insights from RBDCOV Community Advisory Panel Members

Community-Aware Imagery
Representation matters. Tone and emotional impact matter. Choose your images and visuals wisely.
People aspire to see themselves and connect with their experiences in the imagery a project chooses to use. Avoid medicalised imagery. Avoid dark and negative cues and connotations.
These are some examples of inclusive photos, reflecting the diversity of people living with or affected by HIV and other health conditions.
They are sourced from royalty-free image banks, ready to use for campaigns, news, presentations, and educational work.

RBDCOV Expert Articles

FAQs: Understanding the RBDCOV Trials

Glossary of Terms: Vaccine Research & Trials

RBDCOV Talks: The Podcast Series

HIPRA-HH-4 Clinical Trial | Results Report: Participants’ Experience Assessment Study
Psychosocial Study Report

Lay Language Summary on the HIPRA-HH-4 Clinical Trial

Community Engagement Campaign: “It Starts and Ends with Us: Community at the Centre of Clinical Studies”
Real voices. Real stories. This video and blog campaign features RBDCOV Community Advisory members, EATG staff, and a principal investigator sharing their perspectives about trust, representation, and the power of inclusive research.
The campaign emphasises the central role that communities play in making research more ethical, inclusive, and responsive to real needs.
By spotlighting lived experiences and personal reflections, “It Starts and Ends with Us” calls for a shift in how clinical trials are designed and communicated, putting people, not just data, at the heart of the process.
Get to the campaign.


Science Café: Bridging Science and Society on Vaccine Research
Leave No One Behind! Fostering Inclusion in Clinical Trials.
Held on 18 November 2024 in Barcelona and online, this Science Café event aimed to bring science closer to society by creating an informal and welcoming space for open dialogue between researchers and community members.
Participants exchanged ideas on vaccine research and other scientific topics that matter to their daily lives.
Watch the recording (dubbed in English)

Credits
Apostolos Kalogiannis, EATG Communication Manager – Editor-in-Chief & Toolbox Producer
Giorgio Barbareschi, EATG Programme Manager – Toolbox Oversight and Strategic Supervision
Tania Sanchis, EATG Communication Officer – Toolbox Developer
Shatyam Issur, EATG Project Coordinator – Toolbox Coordinator
Rocco Pignata, EATG Programme Officer & Project Coordinator – Content Developer
Fiona Greenhalgh, EATG Programme Officer & Project Coordinator – Content Developer
RBDCOV Community Advisory Panel: Juan Francisco Cabrera Solano, Jennifer Catherine Camaradou, Paul Clift, Marine (Maka) Gogia, Bogdan Hadarag, Apostolos Kalogiannis, Arda Karapınar, Mercy Nangwale, Daniela Rojas Castro, Maryan Said, Siegfried Schwarze, Mona Sundnes, Joan Tallada, Deniz Uyanik, Alain Volny-Anne, Brian Charles West – Content Strategists and Reviewers
Laura Sesma, Health Team Leader & European Programmes (Zabala Innovation) – Toolbox Oversight
Nora Franco, European Projects Dissemination & Communication Leader (Zabala Innovation) – Content Developer & Toolbox Oversight
[Last update: 6 November 2025]
The RBDCOV project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 101046118
Views and opinions expressed are those of the author(s) only and do not necessarily reflect those of the European Union. Neither the European Union nor the granting authority can be held responsible for them.
Get involved
Are you living with HIV/AIDS? Are you part of a community affected by HIV/AIDS and co-infections? Do you work or volunteer in the field? Are you motivated by our cause and interested to support our work?
Subscribe
Stay in the loop and get all the important EATG updates in your inbox with the EATG newsletter. The HIV & co-infections bulletin is your source of handpicked news from the field arriving regularly to your inbox.